Which of the Following Key Ethical Decisions Would an Institutional Review Board (Irb) Approve of?
Upstanding Guidelines for Human Inquiry
Upstanding guidelines in psychological research serve to minimize harm to participants' mental and physical well-being.
Learning Objectives
Justify the need for ethical guidelines in psychological research
Key Takeaways
Fundamental Points
- Ethical guidelines that govern the use of homo subjects in research are a fairly new but of import construct developed in response to unethical and harmful experiments such as the Tuskegee syphilis experiment.
- As a result of various unethical experiments carried out in the United States in the 20th century, several organizations were put in place to assistance monitor clinical inquiry involving humans.
- At nigh colleges and universities, institutional review boards (ethics committees) are formally chosen to approve, review, and monitor bio-medical and behavioral research involving humans.
- Key ethical guidelines include the assurance of confidentiality, informed consent, and debriefing.
Key Terms
- ethical: Of, or relating to, the accepted principles of right and wrong specially those of some organization or profession.
Psychological research involving human being subjects must take into account many ethical considerations. Upstanding guidelines that govern the use of human being subjects in inquiry are a fairly new but of import construct; these ethical policies serve to minimize harm to human participant's mental and physical well being during experimental research.
The Nuremberg Code
An early on instance of the discussion about ethics in inquiry was the Nuremburg Code. The Nuremberg Trials were a series of 12 trials of men defendant of committing war crimes and atrocities during World War Two; amidst those on trial were doctors who had committed crimes confronting humanity such as involuntary human experimentation, involuntary sterilizations, and mass murder under the guise of euthanasia. One upshot of these trials was the Nuremberg Code, a listing of principles for ethical experimentation that included informed consent, absence of coercion, and properly formulated scientific experimentation.
Controversial Experiments
Throughout the 20th century, there were medical and psychological experiments carried out in the U.S. that generated controversy, then outrage, once they were revealed to the general public (oft many years after their conclusion).
Tuskegee Experiment
One of the most infamous instances of unethically-performed experiments was the Tuskegee experiment. From 1932 to 1972, the U.Southward. Public Wellness Service sought to report the natural progression of untreated syphilis in poor, rural black men who idea they were receiving free health care from the U.S. government. Out of the 600 men involved in the experiment, 399 had previously contracted syphilis earlier the study; they were never told they had syphilis, notwithstanding, and were led to believe they were receiving free general medical care. One of the most unethical aspects of the experiment was that past 1947, penicillin was widely recognized as the standard treatment for syphilis. Merely the African American men involved in the experiment were not given the treatment that could cure them, and continued to be studied for 25 years later a cure had been constitute. By the end of the study in 1972, just 74 of the examination subjects were notwithstanding live.
Milgram's Obedience Experiments
The 1961 Milgram experiments examining obedience to authority figures was a notable series of social psychology experiments conducted by Yale University psychologist Stanley Milgram. The experiments measured the willingness of study participants to obey an authorisation figure who instructed them to perform acts that conflicted with their personal censor. Participants were asked past the "authority figure" to human activity as "teachers" and teach "learners" a particular sequence. The authority figure and learner were both in on the experiment, in which the teacher (the experiment subject) was told that the first time the learner made a mistake in the sequence, the teacher had to administer an electric stupor to the learner. The teacher was told to increment the shock for each subsequent mistake the learner fabricated, no matter how much the learner suffered. While the learner never really received an electrical shock, and faked being in pain, the teacher believed that he or she was really shocking the learner. The goal of the study was to come across how far people would go, how high a shock they would deliver, if encouraged past the authority figure to do so. The experiments were controversial and considered past many to be abusive.
Zimbardo's Prison Experiment
The 1971 Stanford prison experiment was a report regarding the psychological effects of becoming a prisoner or prison guard. The participants adapted to their roles well beyond expectations, as the guards enforced authoritarian measures, and ultimately subjected some of the prisoners to psychological torture. Many of the prisoners passively accepted psychological abuse and, at the asking of the guards, readily harassed other prisoners who attempted to prevent it. The experiment fifty-fifty affected the caput researcher himself, who, in his role as the superintendent, permitted the abuse to continue until the experiment ended after only half dozen days. Both guards and prisoners stepped beyond the boundaries of predicted beliefs, leading to dangerous and psychologically damaging situations.
Ethics Organizations
As a outcome of these and other unethical research studies, many organizations have been put in place to help monitor clinical inquiry involving humans. Such organizations include the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, and the Office for Human being Enquiry Protections. At about colleges and universities, ideals committees chosen institutional review boards (IRBs) are formally chosen to corroborate, review, and monitor bio-medical and behavioral research involving humans. IRBs oftentimes deport some form of risk-benefit analysis in an attempt to determine whether or not research should be done, and must approve any experiments done within the organizations they represent.
Ethical Guidelines
To protect the rights and well-being of research participants, and at the aforementioned time discover meaningful results and insights into human behavior, most all psychological inquiry must pass an upstanding review process. At most colleges and universities, this is conducted by the IRB. This grouping examines the proposed research to make sure that no harm is washed to the participants, and that the benefits of the study outweigh any possible risks or discomforts to people taking office in the written report. Minors are more protected than adults in upstanding guidelines, considering a small-scale is not considered to be able to requite fully informed consent.

Right or wrong decision: Upstanding guidelines assist researchers brand the correct decisions, such equally getting informed consent from human subjects.
There is a duty to protect the rights of people in the report besides as their privacy and sensitivity. The confidentiality of those involved in the study must be maintained, keeping their anonymity and privacy secure. A process of informed consent is used to brand sure that volunteers know what will happen in the experiment and understand that they are immune to quit the experiment at any time. Also, a debriefing is typically washed at the decision of the experiment in order to reveal whatever deceptions used and generally make sure that the participants are unharmed by the procedures. Today, most research in social psychology involves no more take chances of impairment than can be expected as by routine psychological testing or normal daily activities.
Ethical Guidelines for Animal Research
Animal research raises the controversial question of whether it is ethical to impairment animals with the aim of improving human lives.
Learning Objectives
Depict the central ethics around psychological research involving animals
Key Takeaways
Key Points
- Whether researchers are working with homo or animal subjects, their experiments ' design and application must be ethical; however, how to define "upstanding" across species is the discipline of much debate.
- While a person can consent to participating in enquiry after being informed of its goals and methods (and this is in fact a mandatory guideline for ethical research among humans), this is non possible for animals.
- From a purely economical standpoint, many debate that animal enquiry is more affordable and economically sound than enquiry conducted on humans.
- Two central questions well-nigh the ethics of animal testing are whether animals have rights and, if they do, whether those rights should be protected.
- The principles of replacement, reduction, and refinement are used to guide more ethical employ of animals in testing and inquiry.
Key Terms
- ethical: Of or relating to the accepted principles of right and incorrect, especially those of some organization or profession.
Controversies in Animal Testing
The use of animals in research is a very controversial topic in today'southward scientific community. While animal research was one time common and unquestioned, information technology now raises an important ethical issue: is it ethical to harm animals with the aim of improving human lives? An experiment's pattern and awarding must exist upstanding whether the enquiry subjects are humans or animals, but how "ethical" is divers across species is the subject of much debate.
A primal departure between an animal and a homo is that animals cannot provide informed consent to participate in an experiment because they cannot understand the risks or consequences of the experiment. While a person tin can consent to participate in research later on beingness informed of an experiment's goals and methods (and in fact this is a mandatory guideline for ethical research among humans), this is not possible for animals, which raises complicated questions about ethics.
The Animal Rights Debate
2 chief questions virtually the ethics of animal testing are whether animals have rights and, if they do, whether those rights should be protected. A legal right is a constabulary-based entitlement that applies to all members of a particular group and is upheld by the justice system. Those in favor of extending equal rights to animals fence that the suffering and well-being of other species are just as of import as the suffering and well-being of humans and should be treated accordingly. It is known that animals can feel pain and distress, and therefore many consider the human action of subjecting animals to pain, injury, or decease for the sake of science to be immoral.
Others argue against extending equal rights to animals, positing that human involvement should be placed above the well-beingness of animals. Many argue that creature research has yielded substantial benefits to the human race, and that these outweigh the negative effects on animals.
Electric current Brute Research
The Animate being Welfare Act (AWA) of 1966 is the only federal law in the United States regulating the treatment of animals in research; while another laws and policies may include additional species coverage or specifications for animal care and utilise, all refer to the AWA as the minimally acceptable standard for fauna treatment and care. Some of the animals covered under the AWA include any live or dead true cat, dog, hamster, rabbit, nonhuman primate, or guinea pig. Animals excluded from this deed are birds, rats, mice, farm animals, and cold-blooded animals.
Under the AWA, all animal dealers must exist registered and licensed, and all animal testing facilities in compliance with this act are required to establish a special committee that includes at least one person trained as a veterinarian and i person who is non affiliated with the facility. These committees regularly appraise animal care, treatment, and practices during research. In improver to compliance with the Brute Welfare Act, most inquiry institutions have an institutional review board (IRB), which is a committee that has been formally designated to approve, monitor, and review biomedical and behavioral research involving humans. About studies involving humans must pass IRB approving before they tin begin.
A diverseness of animals are used in experiments. While animals with shorter life spans and less sophisticated nervous systems tend to be used, this is not always true. Some abet that in that location should be a bureaucracy of animal rights, with more than rights granted to sophisticated species, while others argue that the same rights should be awarded to all living beings.
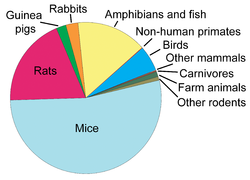
Animal Testing by the Numbers: The proportion of animals used in research testing in Europe in 2005. Mice and rats were the most often used animals.
Replacement, Reduction, and Refinement
Replacement, reduction, and refinement (also referred to as "the three Rs") exist equally guiding principles for more than ethical utilise of animals in testing and research. These guidelines are intended to improve animal welfare and scientific quality where the employ of animals in experimentation cannot be avoided and are implemented in many enquiry labs worldwide. Replacement refers to the preferred use of not-animate being methods whenever it is possible to achieve the aforementioned scientific goals as animal research. Reduction refers to methods that enable researchers to obtain comparable levels of information from fewer animals, or to obtain more data from the same number of animals. Methods of experimental refinement aim to alleviate or minimize potential pain, suffering, or distress and enhance the welfare of the animals used.
Source: https://courses.lumenlearning.com/boundless-psychology/chapter/ethical-considerations-in-research/
0 Response to "Which of the Following Key Ethical Decisions Would an Institutional Review Board (Irb) Approve of?"
Enregistrer un commentaire